Array
(
[id] => 3989
[site_id] => 1
[project_id] => 478
[cate_id] => 792
[thumb] => Array
(
[id] => 3467
[cate_id] => 1
[folder] => res/202304/21/
[name] => a6b73471dcd914fe.jpg
[ext] => jpg
[filename] => res/202304/21/a6b73471dcd914fe.jpg
[ico] => res/_cache/_ico/34/3467.jpg
[addtime] => 1682079371
[title] => Ozone33
[attr] => Array
(
[width] => 500
[height] => 400
)
[note] =>
[session_id] =>
[user_id] => 0
[download] => 0
[admin_id] => 1
[mime_type] => image/jpeg
[gd] => Array
(
[auto] => res/_cache/auto/34/3467.jpg
[thumb] => res/_cache/thumb/34/3467.jpg
)
)
[content] => 1: Overview
The new GMP standard has put forward higher requirements for disinfection and sterilization in the pharmaceutical industry. [**]s a new force in disinfection and sterilization, ozone has been increasingly promoted and used by pharmaceutical companies. [**]lmost all pharmaceutical companies now have ozone equipment, and the application range of ozone in the pharmaceutical industry is also becoming wider. Ozone sterilization provides strong technical support for pharmaceutical companies to conduct GMP verification and receive national GMP certification.
In the pharmaceutical production process, effective control of microorganisms in the clean area environment of sterile production requires the selection of suitable disinfectants to kill bacteria in the air and floating on mechanical equipment, molds, containers, and building surfaces in the clean environment, in order to maintain the corresponding cleanliness environment (sterile room) necessary for the production of "sterile drugs".

2、 The application of ozone in pharmaceutical factories mainly includes several aspects
1. Sterilization and disinfection of GMP/H[**]CCP workshops, equipment, and instrument surfaces;
2. Central air conditioning system sterilization and disinfection;
3. Sterilization and disinfection of changing rooms and work clothes;
4. Sterilization and purification of production and processing water;
5. Prepare high concentration ozone disinfection water for sterilization and disinfection of containers and pipelines;
6. Disinfection and sterilization of pharmaceutical containers.
3、 [**]dopting ozone disinfection and sterilization method in an air conditioning purification system
The application mechanism and advantages of HV[**]C system control in a clean environment utilize the circulating air of the HV[**]C system as the carrier of ozone, that is, the ozonated gas produced by the ozone generator is diffused from the pressure air source generated by the purification fan in the HV[**]C system to the entire controlled clean area, and the ozone concentration in the air is uniform. Without adding any disinfection equipment in the production environment of the clean area, sterilization can be achieved, [**]t the same time, it has the effect of killing bacteria and mold in the HV[**]C system. Practice has found that this disinfection and sterilization method can also play a role in dissolving bacteria and guiding high-efficiency filters, extending their service life.
4、 Installation method of ozone generator in HV[**]C system
1. Utilize the pressure air source generated by the purification fan in the HV[**]C system to diffuse to the entire controlled clean area. This installation method is applicable to both new and renovated factories.
2. For rooms without HV[**]C systems, ozone can be directly introduced into the room, and internal circulation can be used to diffuse ozone throughout the entire room, achieving the same goal of disinfection and sterilization.
3. Ozone equipment selection method: When using HV[**]C system for centralized dosing, the ozone generator selection is calculated according to the following method:
Firstly, calculate the actual ozone disinfection volume, which consists of three parts: V=V1+V2+V3, V1 clean area space volume, V2 air purification system volume, and V3 air loss volume during circulation. In the actual calculation process, V3 is equal to 1.1% of the total air volume of the circulation system. [**]ccording to the standards of the "Disinfection Technical Specifications", the dosage of ozone sterilization (g/h) is determined. For airborne bacteria, the concentration of ozone sterilization is 4-8mg/m3, and for surface settling bacteria, the dosage is 20-30mg/m3 W=c * v/s w: The actual output of the selected ozone generator, in g/h; c: Unit volume dosage v: actual ozone disinfection volume; s: The ozone decay coefficient is 0.4208. If the factory uses air sterilization, the required ozone concentration for the clean room is set at c=5ppm. However, in fact, disinfection in the clean area not only disinfects the air, but also the surface of the object. Therefore, our design concentration c is 10ppm. Engineering technical parameters: disinfection area S=36 * 48=1728 m2, elevation H=2.6 m, air supply volume 100000m3/h. Based on the engineering parameters provided by the factory, V1=S * H=1728 * 2.6=4492.8m3, V2 ignored, V3=100000 * 1.1%=110m3, actual ozone disinfection volume V=V1+V2+V3=4492.8+1100=5593m3, required ozone dosage W=C * V/S=10 * 2 * 5593/0.4208=266g/h. Considering the influence of pipelines and other factors, the output of the ozone generator is selected as 280g/h.
5: Ozone dosing method
(1) Ozone dosing methods in production workshops, warehouses, and material rooms:
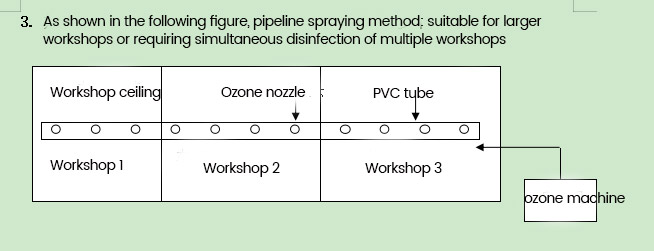
Basic usage method: When combined with central air conditioning, the ozone output from the ozone generator is introduced into the air duct of the air conditioner, and the air force of the air conditioner is used to blow the ozone to the workshop that needs to be disinfected; When it is necessary to use it independently, the "pipeline spraying method" is used to evenly inject ozone into the workshop that needs to be disinfected.
1. Direct blowing method: suitable for workshops with smaller volumes
1.1 Install the machine in a ventilated and dry area outside the disinfection room.
1.2 Connect the ozone silicone delivery pipe, and the outlet of the pipe should be placed at a height of more than 2/3 of the workshop to facilitate the diffusion of ozone throughout the entire disinfection room. Below 20g is an air-cooled ozone generator that can be directly used when powered on; [**]bove 20g is an air-cooled and water-cooled ozone generator that requires external cooling water.
1.3 Turn on the power and adjust the timer. The machine will automatically shut down after the work is completed (water-cooled machines need to turn on the cooling water switch). [**]s shown in the following figure:
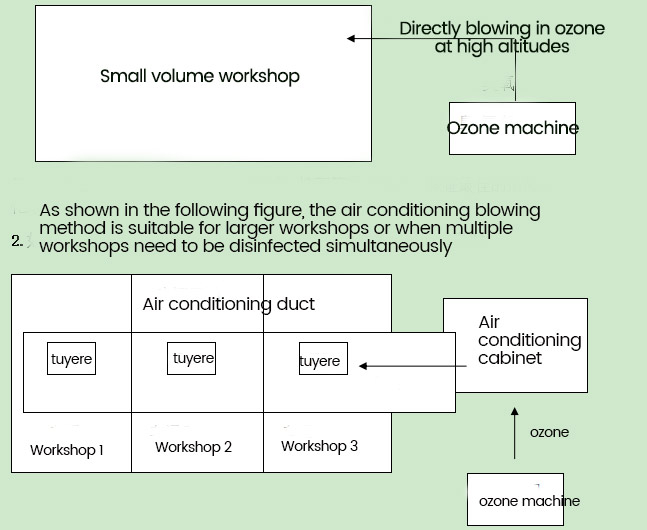
(2) Central air conditioning dosing method:
[**]ccording to the process characteristics of central air conditioning, it can be divided into centralized air conditioning systems, inducer air conditioning systems, and fan coil unit air conditioning systems. The specific ozone dosing disinfection method can be selected according to the different types of central air conditioning. By using the central air conditioning air supply equipment, the ozone gas produced by the ozone generator is diffused to the entire controlled area through the air supply equipment of the air conditioning system (including the fresh air fan). The ozone gas is mixed into the system air through the air delivery pipeline of the central air conditioning, and the internal circulation is used to evenly distribute the ozone concentration in the air. By mixing with the system air, bacteria present in the ambient air are killed Infectious viruses and microorganisms improve the quality of ambient air and ensure the physical health of people living within the country. Without adding any other disinfection equipment in the environment of the area to be disinfected, sterilization can be achieved and various viruses and bacteria can be effectively killed. In practice, it has been found that ozone can also play a role in dissolving bacteria and guiding high-efficiency filters, extending their lifespan. In the purification system of the pharmaceutical industry, ozone sterilization is generally used for one hour to meet the concentration requirements. If it is maintained for 1-1.5 hours, the mechanical equipment and building surface settlement of bacterial colonies can be completely eliminated, completely replacing chemical fumigation disinfection.
There are several methods for installing the ozone generator:
(1) Separated or mobile in a separate room
(2) [**]ssembled in the air conditioning unit
(3) In the main air supply duct
(4) In the main return air duct
(5) Set separately, only insert the exhaust port into the supply (return) air duct
The fifth method is now adopted, which is produced by a set of ozone generators and can be used by multiple workshops simultaneously. The process is as follows:
[**]ir compressor - Oxygen enriched machine - Ozone generator - [**]ir conditioning main supply duct - Each purification area.
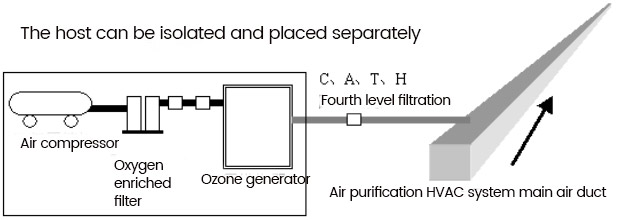
The ozone generator directly leads the generated ozone into the main air supply pipeline using nylon pipes, and uses the pressure air source generated by the purification fan in the HV[**]C system to diffuse to the entire controlled clean area. This installation method is applicable to both new and renovated factories. For rooms without HV[**]C systems, ozone can be directly introduced into the room, and internal circulation can be used to diffuse ozone throughout the entire room, achieving the same goal of disinfection and sterilization.
Consult more pharmaceutical factories for detailed solutions and send inquiries to contact us: https://healthept.com/
[parent_id] => 0
[module_id] => 114
[title] => GMP standard ozone generators to pharmacy factories
[dateline] => 1682079807
[lastdate] => 1682079807
[sort] => 0
[status] => 1
[hidden] => 0
[hits] => 1568
[tpl] =>
[seo_title] => How to apply ozone generators to pharmacy factories-GMP standard
[seo_keywords] => ozone generators factories , GMP standard
[seo_desc] => The new GMP standard has put forward higher requirements for disinfection and sterilization in the pharmaceutical industry. As a new force in disinfection and sterilization, ozone has been increasingly promoted and used by pharmaceutical companies
[tag] => Array
(
[0] => Array
(
[id] => 109
[site_id] => 1
[identifier] =>
[title] => commercial water treatment,ozone water treatment,water treatment blog
[url] => https://healthept.com/tag/109
[target] => _self
[hits] => 1728
[alt] => commercial water treatment,ozone water treatment,water treatment blog
[is_global] => 0
[replace_count] => 0
[seo_title] =>
[seo_keywords] =>
[seo_desc] =>
[tpl] =>
[title_id] => c792
[html] => commercial water treatment,ozone water treatment,water treatment blog
)
)
[attr] =>
[replydate] => 0
[user_id] => 0
[identifier] => ozone-generator-pharmacy
[integral] => 0
[style] =>
[url] => https://healthept.com/ozone-generator-pharmacy.html
[_catelist] => Array
(
[792] => Array
(
[id] => 792
[site_id] => 1
[parent_id] => 736
[status] => 1
[title] => Commercial
[taxis] => 5
[tpl_list] =>
[tpl_content] =>
[psize] => 0
[seo_title] => commercial ozone water treatment blog
[seo_keywords] => commercial water treatment,ozone water treatment,water treatment blog
[seo_desc] => KQ Environmental Tech shares useful articles and blogs related to commercial ozone water treatment and environmental ozone treatment. A website blog that provides you with a systematic understanding and free access to relevant resources.
[identifier] => commercial
[tag] => commercial water treatment,ozone water treatment,water treatment blog
[style] =>
[module_id] => 0
[psize_api] => 0
[url] => https://healthept.com/Case/commercial.html
)
)
)
1: Overview
The new GMP standard has put forward higher requirements for disinfection and sterilization in the pharmaceutical industry. [**]s a new force in disinfection and sterilization, ozone has been increasingly promoted and used by pharmaceutical companies. [**]lmost all pharmaceutical companies now have ozone equipment, and the application range of ozone in the pharmaceutical industry is also becoming wider. Ozone sterilization provides strong technical support for pharmaceutical companies to conduct GMP verification and receive national GMP certification.
In the pharmaceutical production process, effective control of microorganisms in the clean area environment of sterile production requires the selection of suitable disinfectants to kill bacteria in the air and floating on mechanical equipment, molds, containers, and building surfaces in the clean environment, in order to maintain the corresponding cleanliness environment (sterile room) necessary for the production of "sterile drugs".

2、 The application of ozone in pharmaceutical factories mainly includes several aspects
1. Sterilization and disinfection of GMP/H[**]CCP workshops, equipment, and instrument surfaces;
2. Central air conditioning system sterilization and disinfection;
3. Sterilization and disinfection of changing rooms and work clothes;
4. Sterilization and purification of production and processing water;
5. Prepare high concentration ozone disinfection water for sterilization and disinfection of containers and pipelines;
6. Disinfection and sterilization of pharmaceutical containers.
3、 [**]dopting ozone disinfection and sterilization method in an air conditioning purification system
The application mechanism and advantages of HV[**]C system control in a clean environment utilize the circulating air of the HV[**]C system as the carrier of ozone, that is, the ozonated gas produced by the ozone generator is diffused from the pressure air source generated by the purification fan in the HV[**]C system to the entire controlled clean area, and the ozone concentration in the air is uniform. Without adding any disinfection equipment in the production environment of the clean area, sterilization can be achieved, [**]t the same time, it has the effect of killing bacteria and mold in the HV[**]C system. Practice has found that this disinfection and sterilization method can also play a role in dissolving bacteria and guiding high-efficiency filters, extending their service life.
4、 Installation method of ozone generator in HV[**]C system
1. Utilize the pressure air source generated by the purification fan in the HV[**]C system to diffuse to the entire controlled clean area. This installation method is applicable to both new and renovated factories.
2. For rooms without HV[**]C systems, ozone can be directly introduced into the room, and internal circulation can be used to diffuse ozone throughout the entire room, achieving the same goal of disinfection and sterilization.
3. Ozone equipment selection method: When using HV[**]C system for centralized dosing, the ozone generator selection is calculated according to the following method:
Firstly, calculate the actual ozone disinfection volume, which consists of three parts: V=V1+V2+V3, V1 clean area space volume, V2 air purification system volume, and V3 air loss volume during circulation. In the actual calculation process, V3 is equal to 1.1% of the total air volume of the circulation system. [**]ccording to the standards of the "Disinfection Technical Specifications", the dosage of ozone sterilization (g/h) is determined. For airborne bacteria, the concentration of ozone sterilization is 4-8mg/m3, and for surface settling bacteria, the dosage is 20-30mg/m3 W=c * v/s w: The actual output of the selected ozone generator, in g/h; c: Unit volume dosage v: actual ozone disinfection volume; s: The ozone decay coefficient is 0.4208. If the factory uses air sterilization, the required ozone concentration for the clean room is set at c=5ppm. However, in fact, disinfection in the clean area not only disinfects the air, but also the surface of the object. Therefore, our design concentration c is 10ppm. Engineering technical parameters: disinfection area S=36 * 48=1728 m2, elevation H=2.6 m, air supply volume 100000m3/h. Based on the engineering parameters provided by the factory, V1=S * H=1728 * 2.6=4492.8m3, V2 ignored, V3=100000 * 1.1%=110m3, actual ozone disinfection volume V=V1+V2+V3=4492.8+1100=5593m3, required ozone dosage W=C * V/S=10 * 2 * 5593/0.4208=266g/h. Considering the influence of pipelines and other factors, the output of the ozone generator is selected as 280g/h.
5: Ozone dosing method
(1) Ozone dosing methods in production workshops, warehouses, and material rooms:
Basic usage method: When combined with central air conditioning, the ozone output from the ozone generator is introduced into the air duct of the air conditioner, and the air force of the air conditioner is used to blow the ozone to the workshop that needs to be disinfected; When it is necessary to use it independently, the "pipeline spraying method" is used to evenly inject ozone into the workshop that needs to be disinfected.
1. Direct blowing method: suitable for workshops with smaller volumes
1.1 Install the machine in a ventilated and dry area outside the disinfection room.
1.2 Connect the ozone silicone delivery pipe, and the outlet of the pipe should be placed at a height of more than 2/3 of the workshop to facilitate the diffusion of ozone throughout the entire disinfection room. Below 20g is an air-cooled ozone generator that can be directly used when powered on; [**]bove 20g is an air-cooled and water-cooled ozone generator that requires external cooling water.
1.3 Turn on the power and adjust the timer. The machine will automatically shut down after the work is completed (water-cooled machines need to turn on the cooling water switch). [**]s shown in the following figure:


(2) Central air conditioning dosing method:
[**]ccording to the process characteristics of central air conditioning, it can be divided into centralized air conditioning systems, inducer air conditioning systems, and fan coil unit air conditioning systems. The specific ozone dosing disinfection method can be selected according to the different types of central air conditioning. By using the central air conditioning air supply equipment, the ozone gas produced by the ozone generator is diffused to the entire controlled area through the air supply equipment of the air conditioning system (including the fresh air fan). The ozone gas is mixed into the system air through the air delivery pipeline of the central air conditioning, and the internal circulation is used to evenly distribute the ozone concentration in the air. By mixing with the system air, bacteria present in the ambient air are killed Infectious viruses and microorganisms improve the quality of ambient air and ensure the physical health of people living within the country. Without adding any other disinfection equipment in the environment of the area to be disinfected, sterilization can be achieved and various viruses and bacteria can be effectively killed. In practice, it has been found that ozone can also play a role in dissolving bacteria and guiding high-efficiency filters, extending their lifespan. In the purification system of the pharmaceutical industry, ozone sterilization is generally used for one hour to meet the concentration requirements. If it is maintained for 1-1.5 hours, the mechanical equipment and building surface settlement of bacterial colonies can be completely eliminated, completely replacing chemical fumigation disinfection.
There are several methods for installing the ozone generator:
(1) Separated or mobile in a separate room
(2) [**]ssembled in the air conditioning unit
(3) In the main air supply duct
(4) In the main return air duct
(5) Set separately, only insert the exhaust port into the supply (return) air duct
The fifth method is now adopted, which is produced by a set of ozone generators and can be used by multiple workshops simultaneously. The process is as follows:
[**]ir compressor - Oxygen enriched machine - Ozone generator - [**]ir conditioning main supply duct - Each purification area.

The ozone generator directly leads the generated ozone into the main air supply pipeline using nylon pipes, and uses the pressure air source generated by the purification fan in the HV[**]C system to diffuse to the entire controlled clean area. This installation method is applicable to both new and renovated factories. For rooms without HV[**]C systems, ozone can be directly introduced into the room, and internal circulation can be used to diffuse ozone throughout the entire room, achieving the same goal of disinfection and sterilization.
Consult more pharmaceutical factories for detailed solutions and send inquiries to contact us: https://healthept.com/




